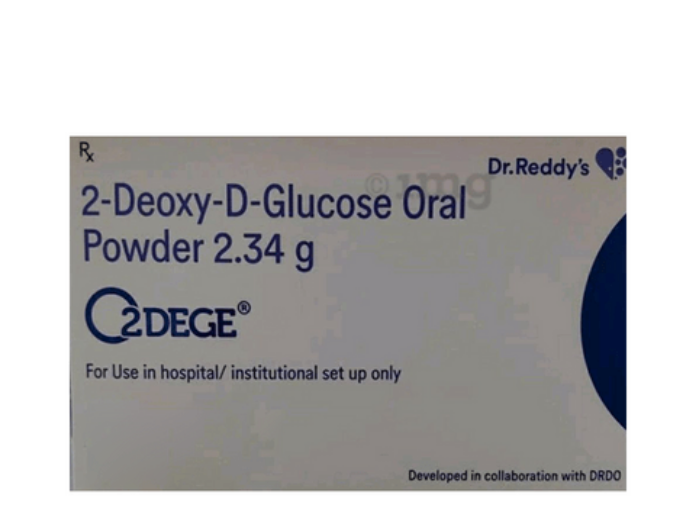
COVID-19 Bajaj Healthcare said in a regulatory filing it “…has received a licence from Defence Research and Development Organisation (DRDO) to Manufacture and Market of ‘2-Deoxy-D-Glucose’ as approved medication for the treatment of COVID-19 patients”.
DRDO has granted Bajaj Healthcare a licence for manufacturing and selling the anti-COVID-19 drugs 2-Deoxy-D-glucose (aka 2DG) on July 7.
In a regulatory filing, Bajaj Healthcare said it “…has received a licence from Defence Research & Development Organisation (DRDO) to manufacture and market 2-deoxy-D-glucose as approved medication for COVID-19 patients”.
In a Live Mint report, Anil Jain, Joint Managing Director at Bajaj Healthcare, said: “We are pleased to add 2-Deoxy-D-Glucose to our growing product portfolio after receiving the license from DRDO. Our country is struggling with a shortage of oxygen capacity. We are hopeful the availability of effective therapies such as 2-DG will ease the pressure and offer patients a timely and much-needed health option.”
The DRDO laboratory — Institute of Nuclear Medicine and Allied Sciences (INMAS) — has developed the 2-DG COVID-19 control and treatment drug with Dr Reddy’s Laboratories.
The Drugs Controller General of India (DCGI) has already approved its use on COVID-19 patients in India. The drug is available in powder form and retails for Rs 990 per sachet.